Critical Limb Ischemia
What is Critical Limb Ischaemia?
Critical Limb Ischaemia (CLI) is an advanced stage of peripheral artery disease. PAD is a narrowing of the arteries in the limbs, typically in the lower legs. CLI results from severely impaired blood flow, which causes pain and tissue damage, including ulceration and gangrene. CLI often results in amputation of the affected limb and is also a major risk factor for cardiovascular events. Around 25% of CLI patients who are unable to undergo vascular surgery to treat the condition die within a year of diagnosis [1].
The data
Cymerus™ MSCs have been successfully tested in a mouse model of CLI. A study conducted by a group of independent scientists at the University of Wisconsin-Madison was published in the prominent peer reviewed journal Cytotherapy, The Journal of Cell Therapy – the official journal of the International Society for Cellular Therapy (ISCT)
In this study, hindlimb ischemia was created in mice by ligating the left common iliac artery and vein, and ligating and severing the femoral artery. Adductor muscles on the ischaemic leg were then injected with either Cymerus MSCs (n=10) or control (n=9) immediately after surgery.
Over a four-week follow-up period, the return of blood flow to the lower limb was measured, using a laser Doppler flow technique. In animals treated with Cymerus MSCs, blood flow in the injured limb was significantly higher at every time point than in animals treated with saline (P<0.006). Moreover, blood flow recovery was faster in the treated animals (P<0.001).
In the series of images of two individual mice. Mouse 1 on the left was treated with Cymerus MSCs, and as can be seen, blood flow (as depicted by a red colour) gradually returns to the injured right hind limb. In contrast, Mouse 2 on the right was treated with saline. The affected limb remains blue (indicating low blood flow), and by day 7 the mouse eventually loses the foot. This can be better seen by the black and white photographs on the far right.
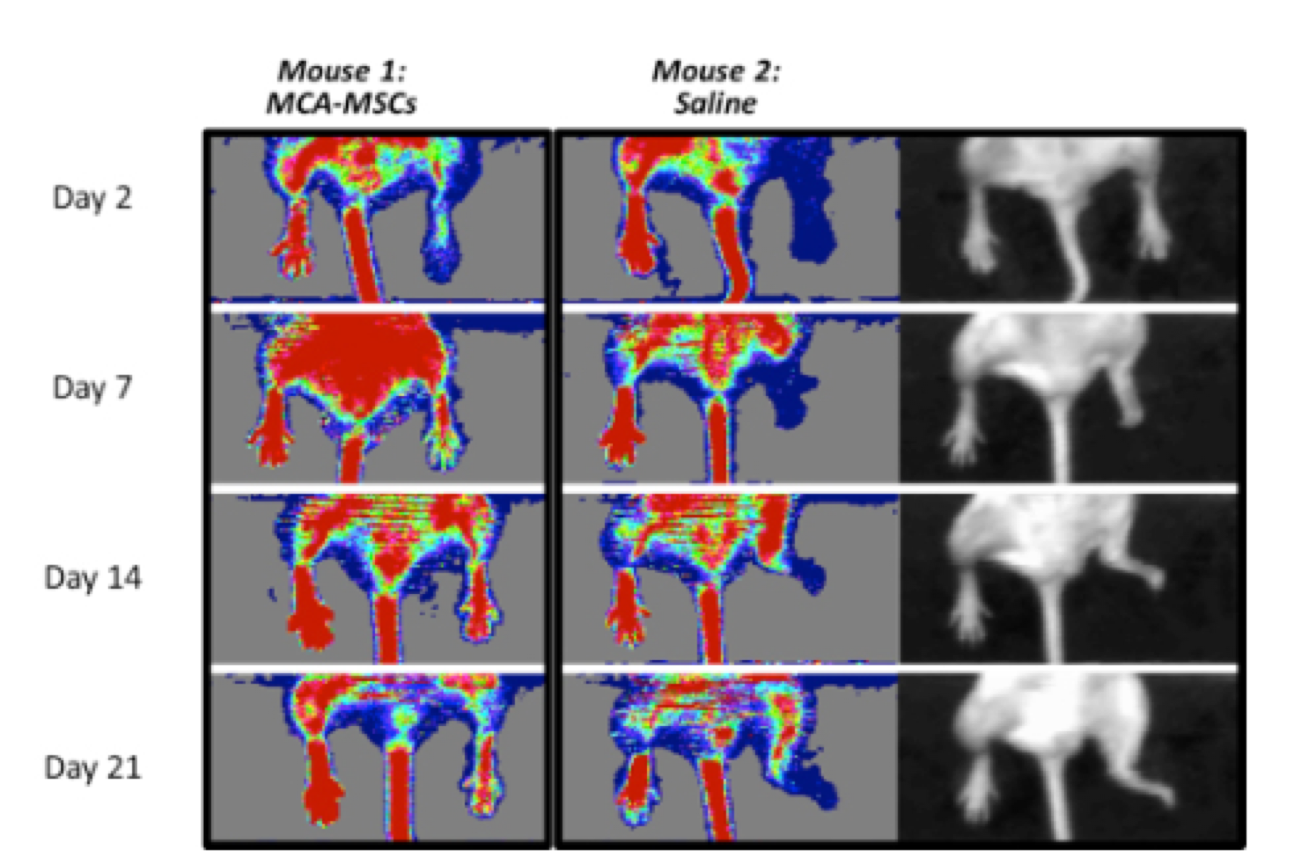
Comparison of Cymerus™-MSCs and saline in blood flow recovery
Histopathology assessments also found significantly less tissue damage in the treated animals, as assessed by myofiber heterogeneity, nuclear centralization, fatty degeneration and fibrosis. The authors of the paper commented “These observations indicate that the extent of improved blood flow observed is likely to be associated with a clinically meaningful benefit”.
In January 2020 we announced the receipt of approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) to proceed with a Phase 2 clinical trial of CYP-002 in patients with (CLI), but in light of the COVID-19 pandemic situation, the decision was taken to put the program on hold.
[1] Norgren L, et al. J Vasc Surg. 2007 Jan;45 Suppl S:S5-67.